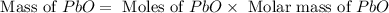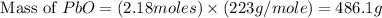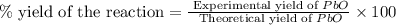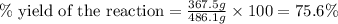Chemistry, 02.03.2020 16:32 StupidFatChipmunk
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g of lead, forming 367.5 g of lead(II) oxide. Calculate the percent yield of the reaction.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

You know the right answer?
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g...
Questions


World Languages, 07.01.2020 18:31

Mathematics, 07.01.2020 18:31

Chemistry, 07.01.2020 18:31

Spanish, 07.01.2020 18:31



History, 07.01.2020 18:31

Social Studies, 07.01.2020 18:31


Mathematics, 07.01.2020 18:31


Spanish, 07.01.2020 18:31

Engineering, 07.01.2020 18:31

English, 07.01.2020 18:31

Mathematics, 07.01.2020 18:31




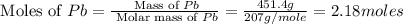
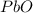
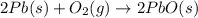
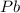 react to give 2 mole of
react to give 2 mole of