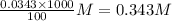Chemistry, 02.03.2020 17:56 trodrickwilliams2019
Determine the molar concentration of ammonium ions, NH4 , in a solution that results when 4.53 g of (NH4)2SO4 are dissolved in 100 mL of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
Determine the molar concentration of ammonium ions, NH4 , in a solution that results when 4.53 g of...
Questions


Physics, 21.03.2021 07:30

Health, 21.03.2021 07:30

Arts, 21.03.2021 07:30

French, 21.03.2021 07:30




Mathematics, 21.03.2021 07:30

Mathematics, 21.03.2021 07:30

Biology, 21.03.2021 07:30

Biology, 21.03.2021 07:30

Mathematics, 21.03.2021 07:30


Mathematics, 21.03.2021 07:30

Mathematics, 21.03.2021 07:30

Medicine, 21.03.2021 07:30

Mathematics, 21.03.2021 07:30


Health, 21.03.2021 07:30

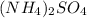 is 132.14 g/mol
is 132.14 g/mol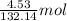 of
of