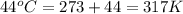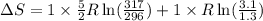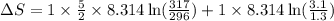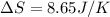Chemistry, 02.03.2020 23:16 camirialchambers17
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simultaneously heated to 44°C. Calculate the entropy change for the process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simult...
Questions








Social Studies, 11.03.2020 21:28

History, 11.03.2020 21:28




Mathematics, 11.03.2020 21:28







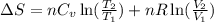
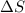 = change in entropy
= change in entropy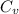 = specific heat capacity at constant volume =
= specific heat capacity at constant volume = 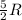 (for monoatomic gas)
(for monoatomic gas)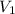 = initial volume of gas = 1.3 L
= initial volume of gas = 1.3 L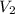 = final volume of gas = 3.1 L
= final volume of gas = 3.1 L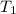 = initial volume of gas =
= initial volume of gas = 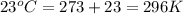
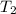 = final volume of gas =
= final volume of gas =