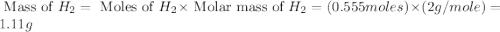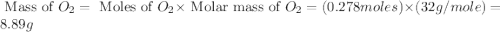Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
What would be the total mass of the products of a reaction in which 10 grams of water decomposes int...
Questions

Social Studies, 17.08.2020 08:01

English, 17.08.2020 08:01


World Languages, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

Biology, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01


Computers and Technology, 17.08.2020 09:01


Mathematics, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01


Chemistry, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

Mathematics, 17.08.2020 09:01

English, 17.08.2020 09:01

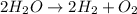
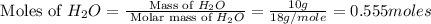
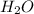 to give 2 mole of
to give 2 mole of 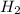
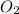
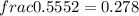 mole of
mole of