
Chemistry, 05.03.2020 03:40 joThompson
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentrations are found to be [PCl5] = 0.40 M, [PCl3] = [Cl2] = 0.20. If the volume of the container is suddenly halved at the same temperature, what will be the new equilibrium concentration of PCl5?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentr...
Questions

Chemistry, 30.06.2019 16:30

Physics, 30.06.2019 16:30


Biology, 30.06.2019 16:30

Mathematics, 30.06.2019 16:30



Physics, 30.06.2019 16:30


Chemistry, 30.06.2019 16:30


History, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30


Biology, 30.06.2019 16:30

Chemistry, 30.06.2019 16:30

History, 30.06.2019 16:30


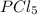 will be 0.9 M.
will be 0.9 M.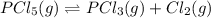
![[PCl_5]=0.40 M](/tpl/images/0534/1154/cb9c6.png)
![[PCl_3]=[Cl_2]=0.20 M](/tpl/images/0534/1154/9bc6a.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0534/1154/73fe0.png)
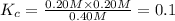
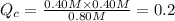
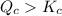
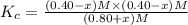
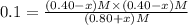
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](/tpl/images/0534/1154/dbad2.png)


