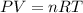Chemistry, 05.03.2020 20:07 nathanscastr02
In preparation for a demonstration, your professor brings a 1.50−L bottle of sulfur dioxide into the lecture hall before class to allow the gas to reach room temperature. If the pressure gauge reads 173 psi and the lecture hall is 20°C, how many moles of sulfur dioxide are in the bottle? In order to solve this problem, you will first need to calculate the pressure of the gas. Hint: The gauge reads zero when 14.7 psi of gas remains.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
In preparation for a demonstration, your professor brings a 1.50−L bottle of sulfur dioxide into the...
Questions







Mathematics, 12.07.2021 07:10

History, 12.07.2021 07:10

History, 12.07.2021 07:10


Mathematics, 12.07.2021 07:10

History, 12.07.2021 07:10


Arts, 12.07.2021 07:10