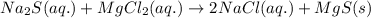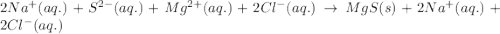Chemistry, 07.03.2020 03:12 foodisbae45678
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chloride. The complete ionic equation contains which of the following species (when balanced in standard form)?a. Cl-(aq)b.2Na+(aq)c.2S2-(aq)d. Na+(aq)e.3Mg2+(aq)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

You know the right answer?
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chlo...
Questions

Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30


Biology, 24.06.2019 12:30

History, 24.06.2019 12:30




English, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30


Mathematics, 24.06.2019 12:30



Mathematics, 24.06.2019 12:30

History, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30

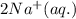 ions
ions