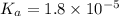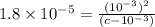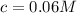Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
A typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous solution of ac...
Questions







Mathematics, 17.05.2021 17:50


Mathematics, 17.05.2021 17:50

Law, 17.05.2021 17:50

English, 17.05.2021 17:50






English, 17.05.2021 17:50


Arts, 17.05.2021 17:50

Arts, 17.05.2021 17:50

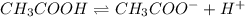
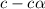
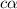
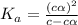
![pH=-log[H^+]](/tpl/images/0539/0805/15713.png)
![3.0=-log[H^+]](/tpl/images/0539/0805/d1dae.png)
![[H^+]=c\times \alpha=10^{-3}](/tpl/images/0539/0805/9452b.png)