Chemistry, 10.03.2020 01:00 jasbutt015p2pqp8
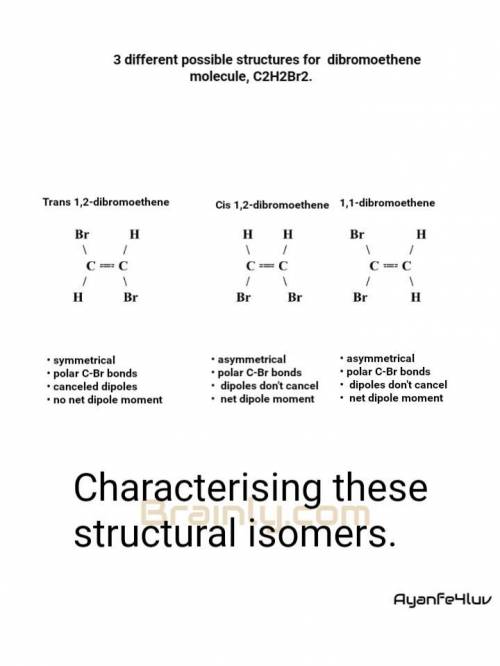
There are three different possible structures (known as isomers) of a dibromoethene molecule, C 2 H 2 Br 2 . One of them has no net dipole moment, but the other two do. Draw Lewis structures for each of these structures. Include H atoms.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
There are three different possible structures (known as isomers) of a dibromoethene molecule, C 2 H...
Questions


Mathematics, 24.12.2019 00:31


French, 24.12.2019 00:31






Computers and Technology, 24.12.2019 00:31










Biology, 24.12.2019 00:31