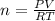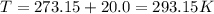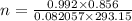Chemistry, 10.03.2020 09:05 lizzyhearts
Calculate the mass of O2 produced by the decomposition of KClO3 when 856 mL of O2 is collected over water at 20.0°C and 1.015 atm. (The vapor pressure of water at 20.0°C is 17.5 torr.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
Calculate the mass of O2 produced by the decomposition of KClO3 when 856 mL of O2 is collected over...
Questions

Chemistry, 02.06.2021 01:00

Mathematics, 02.06.2021 01:00


World Languages, 02.06.2021 01:00

Computers and Technology, 02.06.2021 01:00

Mathematics, 02.06.2021 01:00


Law, 02.06.2021 01:00


English, 02.06.2021 01:00


Spanish, 02.06.2021 01:00

Mathematics, 02.06.2021 01:00



Mathematics, 02.06.2021 01:00

Biology, 02.06.2021 01:00

Mathematics, 02.06.2021 01:00