
Chemistry, 10.03.2020 22:29 chickenwing32
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is the volume of CO2? 1) 57.6 L 2) 22.4 L 3) 0.0250 L 4) 2.80 L 5) 179 L 6) 0.357 L

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is t...
Questions

Mathematics, 29.09.2019 02:30




Mathematics, 29.09.2019 02:30

Physics, 29.09.2019 02:30




Mathematics, 29.09.2019 02:50

Mathematics, 29.09.2019 02:50

Arts, 29.09.2019 02:50

Mathematics, 29.09.2019 02:50





Mathematics, 29.09.2019 02:50


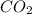 = 8.0 mole
= 8.0 mole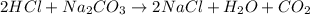
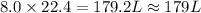 volume of
volume of 

