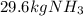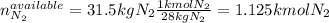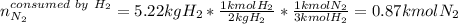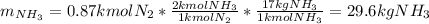Chemistry, 13.03.2020 02:27 ddddre3909
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kg, that we can synthesize from 5.22 kg of H2 and 31.5 kg of N2? Express the mass in kilograms to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 21:30
Which particles make up the nucleus of an atom? a. protons and electrons b. neutrons and electrons c. protons only d. protons and neutrons e. neutrons only
Answers: 1
You know the right answer?
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield...
Questions

Biology, 15.08.2020 19:01

Mathematics, 15.08.2020 19:01


Mathematics, 15.08.2020 19:01

English, 15.08.2020 19:01



Computers and Technology, 15.08.2020 19:01



Computers and Technology, 15.08.2020 19:01

Chemistry, 15.08.2020 19:01