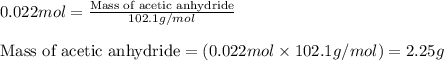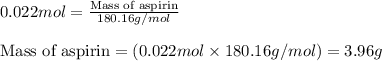Chemistry, 13.03.2020 18:34 chloeann5397
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according to the following equation:
C7H6O3Salicylicacid+C4H6O3Aceticanh ydride→C9H8O4Aspirin+CH3COOHAcetica cid
a. How many grams of acetic anhydride are needed to react with 3.07 g of salicylic acid?
b. How many grams of aspirin will result?
c. How many grams of acetic acid are formed as a by-product?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2


Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2
You know the right answer?
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according t...
Questions




History, 24.12.2019 17:31


History, 24.12.2019 17:31

English, 24.12.2019 17:31










History, 24.12.2019 17:31

English, 24.12.2019 17:31


History, 24.12.2019 17:31

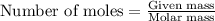 .....(1)
.....(1)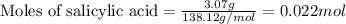

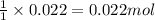 of acetic anhydride
of acetic anhydride