
Chemistry, 13.03.2020 20:03 LilFreaky666
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (aq) + 3 H₂ (g); ΔH₁ = − 878.0 k J
HCl (g) ⟶ HCl (aq); ΔH₂ = − 74.8 k J
H₂ (g) + Cl₂ (g) ⟶ 2 HCl (g); ΔH₃ = − 1845.0 k J
MCl₃ (s) ⟶ MCl₃ ( aq ); ΔH₄ = − 497.0 k J
Use the given information to determine the enthalpy of the reaction
2 M (s) + 3 Cl₂ (g) ⟶ 2 MCl₃ (s).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2


Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
Questions


Spanish, 30.11.2020 22:50

Spanish, 30.11.2020 22:50






Mathematics, 30.11.2020 23:00

Chemistry, 30.11.2020 23:00





History, 30.11.2020 23:00





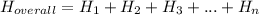 .
.

