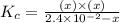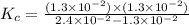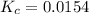Consider the following reaction:
SO2Cl2(g)⇌SO2(g)+Cl2(g)
A reaction mixture is made cont...

Chemistry, 14.03.2020 01:16 Bescobar017
Consider the following reaction:
SO2Cl2(g)⇌SO2(g)+Cl2(g)
A reaction mixture is made containing an initial [SO2Cl2] of 2.4×10−2 M . At equilibrium, [Cl2]= 1.3×10−2 M .
Calculate the value of the equilibrium constant (Kc).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
Questions

Mathematics, 14.07.2021 08:10

Mathematics, 14.07.2021 08:10


Business, 14.07.2021 08:10



English, 14.07.2021 08:10

Mathematics, 14.07.2021 08:10

English, 14.07.2021 08:10

Social Studies, 14.07.2021 08:10

Chemistry, 14.07.2021 08:10

Chemistry, 14.07.2021 08:10


Mathematics, 14.07.2021 08:10

Mathematics, 14.07.2021 08:10


Physics, 14.07.2021 08:10

History, 14.07.2021 08:10


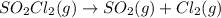
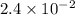 0 0
0 0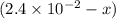 x x
x x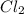 at equilibrium =
at equilibrium = 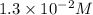
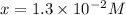
![K_c=\frac{[SO_2][Cl_2]}{[SO_2Cl_2]}](/tpl/images/0547/3049/1e2ab.png)