
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. You determine that the concentration of the unknown acid was 0.1224 M. You also determined that the acid was monoprotic (HA). What is the K_a and pK_a of your unknown acid

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. Yo...
Questions





Mathematics, 15.12.2020 21:30


English, 15.12.2020 21:30



Mathematics, 15.12.2020 21:30


Mathematics, 15.12.2020 21:30

Chemistry, 15.12.2020 21:30



Mathematics, 15.12.2020 21:30


Mathematics, 15.12.2020 21:30


 is dissociation constant and the value of
is dissociation constant and the value of 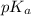 is 4.98.
is 4.98.![pH=-\log[H^+]](/tpl/images/0548/6523/cf945.png)
![2.95=-\log[H^+]](/tpl/images/0548/6523/b4bb5.png)
![[H^+]=10^{-2.95}=0.001122 M](/tpl/images/0548/6523/2ad17.png) ..[1]
..[1]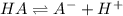
![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0548/6523/a5cb9.png)
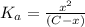
![[H^+]=x =0.001122 M](/tpl/images/0548/6523/90707.png) ( from [1])
( from [1])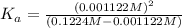
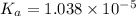
![pK_a=-\log[K_a]](/tpl/images/0548/6523/78bbf.png)
![=-\log[1.038\times 10^{-5}]=4.98](/tpl/images/0548/6523/44380.png)



