
Chemistry, 16.03.2020 20:09 ginareyes0423
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the following reactions and given ΔHΔH values: C3H8(l)+5O2(g)→3CO2(g)+4H2O(g),ΔHC( s)+O2(g)→CO2(g),ΔH2H2(g)+O2(g)→2H2O (g),ΔH===−2026.6kJ−393.5kJ−483.5kJ

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the f...
Questions




English, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00





English, 16.12.2021 05:00


Social Studies, 16.12.2021 05:00

Mathematics, 16.12.2021 05:00


Biology, 16.12.2021 05:00

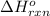 for the reaction is -120.9 kJ.
for the reaction is -120.9 kJ.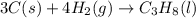
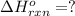
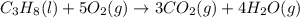
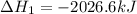
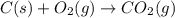
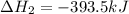 ( × 3)
( × 3)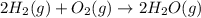
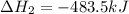 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0548/9833/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times -(-2026.6))+(3\times (-393.5))+(2\times (-483.5))]=-120.9kJ](/tpl/images/0548/9833/4ea2a.png)


