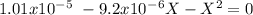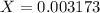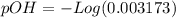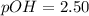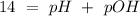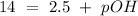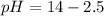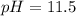Chemistry, 19.03.2020 22:19 nativebabydoll35
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M solution of allantoin at 25°C. Round your answer to 1 decimal pl

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3



Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M...
Questions





Health, 24.04.2020 18:27



Biology, 24.04.2020 18:27

English, 24.04.2020 18:27


Computers and Technology, 24.04.2020 18:27

English, 24.04.2020 18:27

Mathematics, 24.04.2020 18:27



English, 24.04.2020 18:27


Mathematics, 24.04.2020 18:27


Mathematics, 24.04.2020 18:27

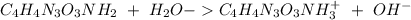
![Kb=\frac{[C_4H_4N_2O_3NH_3^+][OH^-]}{[C_4H_4N_3O_3NH_2]}](/tpl/images/0554/7437/19295.png)
![9.2x10^-^6=\frac{[X][X]}{[1.1-X]}](/tpl/images/0554/7437/d576a.png)
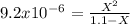
![(9.2x10^-^6)*[1.1-X]=X^2](/tpl/images/0554/7437/e621d.png)