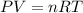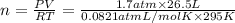Chemistry, 20.03.2020 02:04 harris435942
A cylinder contains 26.5 L of oxygen gas at a pressure of 1.7 atm and a temperature of 295 K. Part A How much gas (in moles) is in the cylinder? Express your answer to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
A cylinder contains 26.5 L of oxygen gas at a pressure of 1.7 atm and a temperature of 295 K. Part A...
Questions

History, 16.12.2020 18:20


Mathematics, 16.12.2020 18:20

Social Studies, 16.12.2020 18:20





History, 16.12.2020 18:20


History, 16.12.2020 18:20


English, 16.12.2020 18:20



Chemistry, 16.12.2020 18:20

History, 16.12.2020 18:20

History, 16.12.2020 18:20

Mathematics, 16.12.2020 18:20

Mathematics, 16.12.2020 18:20