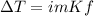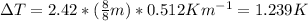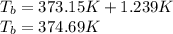Chemistry, 20.03.2020 04:25 nunnielangley0
What will be the boiling point of a solution of 8 moles of sodium dichromate (Na2Cr2O7) dissolved in 8 kg of water? Use the following values: Kb = 0.512 K · m−1 Kf = 1.86 K · m−1 1. 374.69 K 2. 373.92 K 3. 378.73 K 4. 380.27 K 5. 377.76 K

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

You know the right answer?
What will be the boiling point of a solution of 8 moles of sodium dichromate (Na2Cr2O7) dissolved in...
Questions



Biology, 26.11.2020 01:50

Law, 26.11.2020 01:50

Arts, 26.11.2020 01:50

History, 26.11.2020 01:50

Mathematics, 26.11.2020 01:50






Mathematics, 26.11.2020 01:50

Chemistry, 26.11.2020 01:50


Health, 26.11.2020 01:50

Mathematics, 26.11.2020 01:50

Chemistry, 26.11.2020 01:50


History, 26.11.2020 01:50