
Chemistry, 20.03.2020 09:57 nommies005
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl. Write out the dominant equilibrium (including phase labels) that would exist in this "neutralized" solution. HINT: first look at what ions would exist in solution after the non-equilibrium reaction with the strong acid is over. Second, decide if either of these ions is an acid or a base. Lastly, write the equation for this ion acting as an acid or base in water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
4. A solution of the weak base, ammonia (NH3), was completely neutralized with the strong acid HCl....
Questions

Mathematics, 14.07.2019 00:30


English, 14.07.2019 00:30



Mathematics, 14.07.2019 00:30

Health, 14.07.2019 00:30


Biology, 14.07.2019 00:30


Mathematics, 14.07.2019 00:30




Mathematics, 14.07.2019 00:30

English, 14.07.2019 00:30

Biology, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30

Mathematics, 14.07.2019 00:30

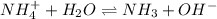
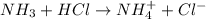
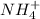 and
and 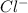 ions exist.
ions exist.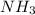 is an weak base and HCl is a strong acid.
is an weak base and HCl is a strong acid.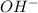 .
.

