Chemistry, 20.03.2020 11:40 ineedtopeebeforethec
A 8.70 L 8.70 L container holds a mixture of two gases at 33 ° C. 33 °C. The partial pressures of gas A and gas B, respectively, are 0.314 atm 0.314 atm and 0.755 atm. 0.755 atm. If 0.200 mol 0.200 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2
You know the right answer?
A 8.70 L 8.70 L container holds a mixture of two gases at 33 ° C. 33 °C. The partial pressures of ga...
Questions


Biology, 19.10.2020 01:01


Mathematics, 19.10.2020 01:01

Mathematics, 19.10.2020 01:01


Social Studies, 19.10.2020 01:01


Mathematics, 19.10.2020 01:01

Mathematics, 19.10.2020 01:01



Computers and Technology, 19.10.2020 01:01


Social Studies, 19.10.2020 01:01



Chemistry, 19.10.2020 01:01


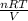 =
= 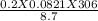 = 0.577 atm
= 0.577 atm