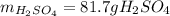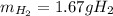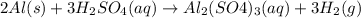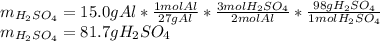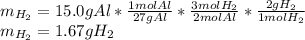Sulfuric acid dissolves aluminum metal according to the following reaction:
2Al (s) + 3H2SO4...

Chemistry, 21.03.2020 05:32 dalton200166
Sulfuric acid dissolves aluminum metal according to the following reaction:
2Al (s) + 3H2SO4 (aq) --> Al2(SO4)3 (aq) + 3H2 (g)
Suppose you wanted to dissolve an aluminum block with a mass of 15.0 g.
What minimum mass of H2SO4 (in g) would you need?
What mass of H2 gas (in g) would be produced by the complete reaction of the aluminum block?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Questions


Mathematics, 13.12.2019 01:31


Geography, 13.12.2019 01:31

Mathematics, 13.12.2019 01:31

English, 13.12.2019 01:31

History, 13.12.2019 01:31




History, 13.12.2019 01:31

Mathematics, 13.12.2019 01:31


English, 13.12.2019 01:31


Mathematics, 13.12.2019 01:31


Mathematics, 13.12.2019 01:31

Mathematics, 13.12.2019 01:31

Mathematics, 13.12.2019 01:31