
Chemistry, 24.03.2020 00:00 paolaviviana
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C. 2 H2S(g) 2 H2(g) + S2(g) If 0.31 mol H2S is placed in a 4.1 L container, what is the equilibrium concentration of H2(g) at 700°C?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C....
Questions

Mathematics, 17.12.2020 21:20




Mathematics, 17.12.2020 21:20

Mathematics, 17.12.2020 21:20




Mathematics, 17.12.2020 21:20

Biology, 17.12.2020 21:20


Mathematics, 17.12.2020 21:20

Mathematics, 17.12.2020 21:20



Mathematics, 17.12.2020 21:20

Arts, 17.12.2020 21:20

Computers and Technology, 17.12.2020 21:20

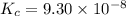 }
}![[concentration]=\frac{moles}{volume (L)}](/tpl/images/0559/9416/7e1bc.png)
![[H_2S]=\frac{0.31 mol}{4.1 L}=0.076 M](/tpl/images/0559/9416/b2f14.png)
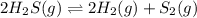
![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0559/9416/3ac5e.png)
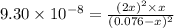
![[H_2]=2x=2\times 0.00051 M=0.0010 M](/tpl/images/0559/9416/46b67.png)


