
Chemistry, 24.03.2020 00:56 arielmajesty9107
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) + 5 I-(aq) + 6 H+(aq) --> 3 I2(aq) + 3 H2O(l) followed by reaction of the I2: I2(aq) + 2 S2O32- --> 2 I-(aq) + S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(...
Questions


Mathematics, 28.10.2020 14:00

Health, 28.10.2020 14:00

English, 28.10.2020 14:00




Mathematics, 28.10.2020 14:00

English, 28.10.2020 14:00



Advanced Placement (AP), 28.10.2020 14:00

Mathematics, 28.10.2020 14:00



Medicine, 28.10.2020 14:00

Business, 28.10.2020 14:00


Computers and Technology, 28.10.2020 14:00

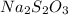 reacting with one mole of
reacting with one mole of 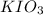 .
.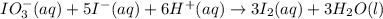 ..[1]
..[1]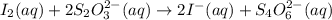 ..[2]
..[2]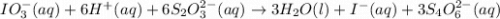
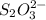 reacts with 1 mole of
reacts with 1 mole of 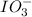 .
.

