Chemistry, 24.03.2020 03:47 HTKPenguin
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 100.0 mL of 0.200 M acetic acid. Assume there is no change in volume on adding the salt to the acid. (pKa for acetic acid is 4.74 or Ka is 1.8 x 10-5)3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 1...
Questions







Mathematics, 28.02.2020 02:02


Biology, 28.02.2020 02:02

Mathematics, 28.02.2020 02:03



Mathematics, 28.02.2020 02:03


Chemistry, 28.02.2020 02:03

Computers and Technology, 28.02.2020 02:03

Mathematics, 28.02.2020 02:03



Mathematics, 28.02.2020 02:03

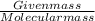
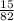
![\frac{[Salt]}{[Acid]}](/tpl/images/0560/4655/6743a.png)