
Chemistry, 25.03.2020 06:41 sophia4636
What will have to happen to the temperature of a sample of methane if 1000 mL at 98.6 kPa and 25°c is given a pressure of 108.5 kPa and a volume of 900 mL?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

You know the right answer?
What will have to happen to the temperature of a sample of methane if 1000 mL at 98.6 kPa and 25°c i...
Questions


History, 22.07.2019 20:30




Mathematics, 22.07.2019 20:30


Arts, 22.07.2019 20:30






Mathematics, 22.07.2019 20:30





History, 22.07.2019 20:30

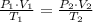
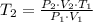 =
= 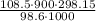 = 295.277 K
= 295.277 K

