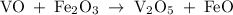Chemistry, 25.03.2020 17:43 carsondelane13
Vanadium (II) oxide with Iron (III) oxide results in Vanadium (V) oxide and Iron (II) oxide. Balance this equation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
Vanadium (II) oxide with Iron (III) oxide results in Vanadium (V) oxide and Iron (II) oxide. Balance...
Questions



Mathematics, 17.06.2021 22:40

Mathematics, 17.06.2021 22:40


Mathematics, 17.06.2021 22:40

Mathematics, 17.06.2021 22:40

Mathematics, 17.06.2021 22:40

English, 17.06.2021 22:40



Spanish, 17.06.2021 22:40





Chemistry, 17.06.2021 22:40

English, 17.06.2021 22:40

Mathematics, 17.06.2021 22:40

Mathematics, 17.06.2021 22:40