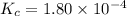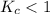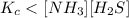Chemistry, 26.03.2020 21:33 demienarravo
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) This reaction is Reactant favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2...
Questions




















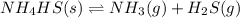
![K_c=[NH_3][H_2S]](/tpl/images/0565/8253/ff5ed.png)
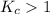 ; the reaction is product favored.
; the reaction is product favored.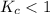 ; the reaction is reactant favored.
; the reaction is reactant favored.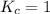 ; the reaction is in equilibrium.
; the reaction is in equilibrium.