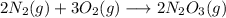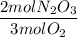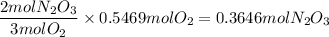Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
In a particular reaction 6.80g of dinitrogen trioxide gas (N203) was actually produced by
reac...
reac...
Questions


Mathematics, 01.09.2021 18:20

Physics, 01.09.2021 18:20


Mathematics, 01.09.2021 18:20



English, 01.09.2021 18:20







History, 01.09.2021 18:20

History, 01.09.2021 18:20

Mathematics, 01.09.2021 18:20

Mathematics, 01.09.2021 18:20