
Chemistry, 30.03.2020 21:03 kharmaculpepper
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is responsible for the acid rain phenomenon. S(s) + O2(g) → SO2(g) If 4.10 kg of S are reacted with oxygen, calculate the volume of SO2 gas (in mL) formed at 30°C and 1.16 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is respo...
Questions















Biology, 05.06.2021 22:50


Mathematics, 05.06.2021 22:50


Business, 05.06.2021 22:50

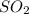 gas is,
gas is, 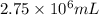
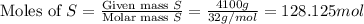
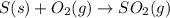
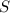 react to give 1 mole of
react to give 1 mole of 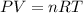
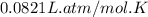
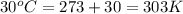
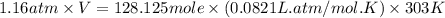
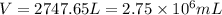 (1 L = 1000 mL)
(1 L = 1000 mL)

