
Chemistry, 30.03.2020 22:42 OGrant18075
Following the instructions in your lab manual, you have titrated a 25.00 mL sample of 0.0100 M KIO3 with a solution of Na2S2O3 of unknown concentration. The endpoint was observed to occur at 16.50 mL. 1. How many moles of KIO3 were titrated? Show work! 2. How many moles of Na2S2O3 did this require?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Following the instructions in your lab manual, you have titrated a 25.00 mL sample of 0.0100 M KIO3...
Questions

Mathematics, 17.04.2021 18:50

World Languages, 17.04.2021 18:50

Mathematics, 17.04.2021 18:50


English, 17.04.2021 18:50

Chemistry, 17.04.2021 18:50

History, 17.04.2021 18:50


History, 17.04.2021 18:50

Mathematics, 17.04.2021 18:50


Physics, 17.04.2021 18:50

English, 17.04.2021 18:50

Chemistry, 17.04.2021 18:50


Health, 17.04.2021 18:50




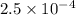 moles
moles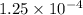 moles
moles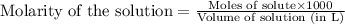
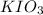 solution = 0.0100 M
solution = 0.0100 M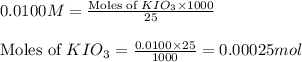
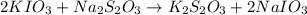
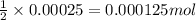 of sodium thiosulfate
of sodium thiosulfate

