Chemistry, 01.04.2020 22:35 glocurlsprinces
The molar solubility of zns is 1.6 Ã 10-12 m in pure water. Calculate the ksp for zns.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
The molar solubility of zns is 1.6 Ã 10-12 m in pure water. Calculate the ksp for zns....
Questions

Mathematics, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00

History, 10.11.2020 01:00

Biology, 10.11.2020 01:00



Mathematics, 10.11.2020 01:00

English, 10.11.2020 01:00

English, 10.11.2020 01:00

Physics, 10.11.2020 01:00

Social Studies, 10.11.2020 01:00

Mathematics, 10.11.2020 01:00





English, 10.11.2020 01:00



Social Studies, 10.11.2020 01:00

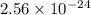 .
.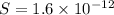
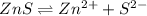
![K_{sp}=[Zn^{2+}]\times [S^{2-}]](/tpl/images/0576/5742/dbee8.png)