
Chemistry, 04.04.2020 12:08 ErrorNameTaken505
Lead (II) sulfide, PbS, reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide. If 0.750 moles of O2 were used during this chemical reaction, how many grams of lead (II) oxide would be produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Lead (II) sulfide, PbS, reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide. If 0.7...
Questions

Mathematics, 29.06.2019 02:00


Mathematics, 29.06.2019 02:00


Mathematics, 29.06.2019 02:00


Mathematics, 29.06.2019 02:00


Social Studies, 29.06.2019 02:00

Mathematics, 29.06.2019 02:00



Mathematics, 29.06.2019 02:00

Social Studies, 29.06.2019 02:00

Chemistry, 29.06.2019 02:00




Chemistry, 29.06.2019 02:00

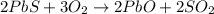
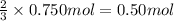 of lead (II) oxide
of lead (II) oxide

