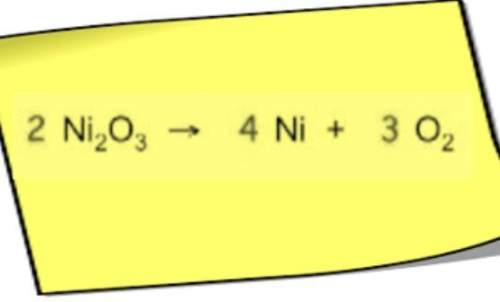
Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Sulfurous Acid, H2SO3, is a diprotic acid, with the following acid dissociation constants: Ka1 = 1.4...
Questions

Arts, 31.05.2020 14:57




History, 31.05.2020 14:57




Biology, 31.05.2020 14:57



Mathematics, 31.05.2020 14:57

Biology, 31.05.2020 14:57



Chemistry, 31.05.2020 14:57




Mathematics, 31.05.2020 14:57