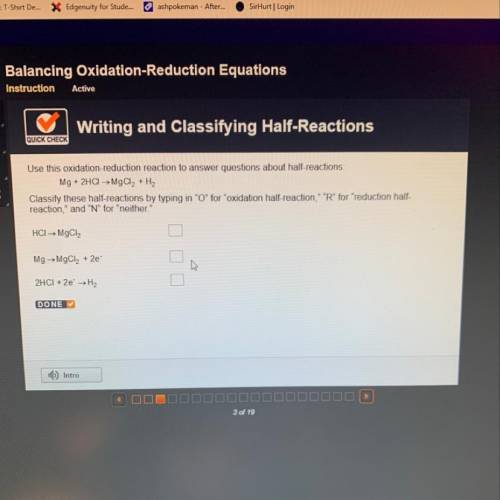
Use this oxidation-reduction reaction to answer questions about half-reactions:
Mg + 2HCl → Mg...

Chemistry, 05.04.2020 04:59 Haleysaraya1
Use this oxidation-reduction reaction to answer questions about half-reactions:
Mg + 2HCl → MgCl2 + H2
Classify these half-reactions by typing in "O" for "oxidation half-reaction," "R" for "reduction half-
reaction," and "N" for "neither."
HCI→ MgCl2
Mg → MgCl2 + 2e
2HCI + 2e →H2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Questions


Mathematics, 05.10.2019 20:10


Biology, 05.10.2019 20:10

History, 05.10.2019 20:10




Geography, 05.10.2019 20:10


Physics, 05.10.2019 20:10


English, 05.10.2019 20:10

Chemistry, 05.10.2019 20:10

Geography, 05.10.2019 20:10

Mathematics, 05.10.2019 20:10

History, 05.10.2019 20:10

Mathematics, 05.10.2019 20:10




