If 25 g of NH3, and 96 g of H2S react according to the following reaction, what is the
maximum...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Questions




Mathematics, 10.12.2021 04:10

Mathematics, 10.12.2021 04:10




Mathematics, 10.12.2021 04:10



History, 10.12.2021 04:10

History, 10.12.2021 04:10


Mathematics, 10.12.2021 04:10

Mathematics, 10.12.2021 04:10


Mathematics, 10.12.2021 04:10

English, 10.12.2021 04:10

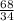 g of (NH₄)₂S
g of (NH₄)₂S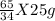 of (NH₄)₂S
of (NH₄)₂S

