
Chemistry, 07.04.2020 03:19 izzybella18ozgsrg
The molar mass of Allura red is 496 g/mol. The stock solution contains 93.84 mg/L Allura red. ac. To create 100.00 mL of a 1.89 x 10-6 M solution, you would need this many mL of the stock solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
The molar mass of Allura red is 496 g/mol. The stock solution contains 93.84 mg/L Allura red. ac. To...
Questions

History, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10


Biology, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10


History, 20.11.2020 22:10

Chemistry, 20.11.2020 22:10

Business, 20.11.2020 22:10


Mathematics, 20.11.2020 22:10




Biology, 20.11.2020 22:10





Biology, 20.11.2020 22:10

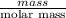
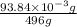
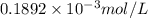
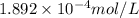
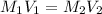
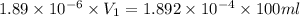
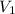 = 1.0 ml
= 1.0 ml

