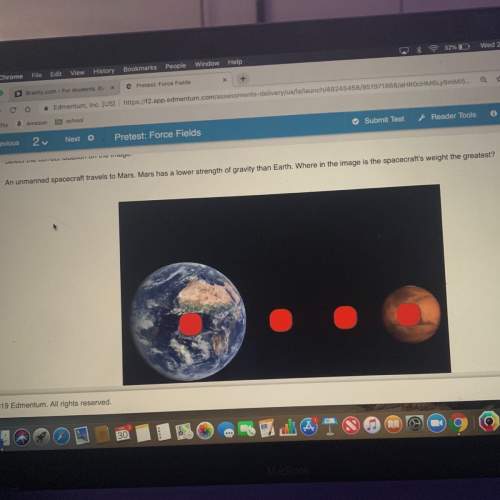
2Be + O2 = 2BeO
a. How many moles of BeO are made by 52.1 grams of Be?
b. How many...

Chemistry, 07.04.2020 07:19 kkelley3141
2Be + O2 = 2BeO
a. How many moles of BeO are made by 52.1 grams of Be?
b. How many grams of O2 will make 3.78 moles of BeO?
c. 245 grams of Be combine with how many moles of O2?
4. Equation: 4 Fe + 3 O2 = 2 Fe2O3
a. How many moles of iron will combine with 2.50 moles of oxygen O2?
b. How many moles of Fe2O3 is produced by 3.01 moles of oxygen O2?
c. 7.61 moles of iron will produce how many grams of Fe2O3?
d. 1650 grams of iron will produce how many moles of Fe2O3?
4. Equation: 4 Li + O2 = 2 Li2O
a. How many moles of lithium combine with 2.95 moles of oxygen O2?
b. How many moles of Li2O are produced from 13.5 moles of lithium?
c. 4.17 moles of oxygen O2 will produce how many grams of Li2O?
d. 5.28 grams of lithium will produce how many moles of Li2O?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
Questions

History, 24.07.2019 23:00

Mathematics, 24.07.2019 23:00


Mathematics, 24.07.2019 23:00

Computers and Technology, 24.07.2019 23:00

Chemistry, 24.07.2019 23:00

Chemistry, 24.07.2019 23:00


Mathematics, 24.07.2019 23:00

History, 24.07.2019 23:00

Mathematics, 24.07.2019 23:00





Mathematics, 24.07.2019 23:00


Business, 24.07.2019 23:00

Social Studies, 24.07.2019 23:00

Mathematics, 24.07.2019 23:00