Chemistry, 07.04.2020 16:08 julianastri6841
3) A saturated solution of PbCl2 in water was prepared and filtered. From the filtrate (solution collected after filtration), 500 mL was measured out into a beaker and evaporated to dryness. The solid PbCl2 residue recovered in the beaker amounted to 2.0 grams. a) Calculate the molar solubility of PbCl2. b) Calculate the solubility product constant, Ksp, for PbCl2

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
3) A saturated solution of PbCl2 in water was prepared and filtered. From the filtrate (solution col...
Questions


Computers and Technology, 22.08.2019 18:30

Computers and Technology, 22.08.2019 18:30





Computers and Technology, 22.08.2019 18:30


Computers and Technology, 22.08.2019 18:30

Mathematics, 22.08.2019 18:30



Health, 22.08.2019 18:40

Health, 22.08.2019 18:40

Mathematics, 22.08.2019 18:40

English, 22.08.2019 18:40



Social Studies, 22.08.2019 18:40

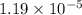
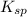
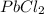 is given as:
is given as: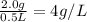
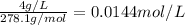
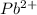 and 2 moles of
and 2 moles of 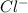 ions
ions![[Pb^{2+}][Cl^-]^2](/tpl/images/0586/4369/716d2.png)
![K_{sp}=[0.0144][2\times 0.0144]^2](/tpl/images/0586/4369/87f57.png)