
Chemistry, 07.04.2020 18:44 needhelpasap4900
Consider the following elementary reaction: CFCl3(g)→CFCl2(g)+Cl(g).Suppose we let k1 stand for the rate constant of this reaction, and k−1 stand for the rate constant of the reverse reaction.
Write an expression that gives the equilibrium concentration of CFCl3 in terms of k1,k−1, and the equilibrium concentrations of CFCl2 and Cl.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

You know the right answer?
Consider the following elementary reaction: CFCl3(g)→CFCl2(g)+Cl(g).Suppose we let k1 stand for the...
Questions





Mathematics, 14.02.2020 04:48

Mathematics, 14.02.2020 04:48




Chemistry, 14.02.2020 04:48



Mathematics, 14.02.2020 04:49





Mathematics, 14.02.2020 04:49


Computers and Technology, 14.02.2020 04:49

![[CFCl_3(g)]=\dfrac{k_{-1}}{k_1}\cdot[CFCl_2(g)]\cdot [Cl(g)]](/tpl/images/0586/7481/11f97.png)
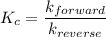
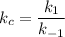
![k_c=\dfrac{[CFCl_2(g)]\cdot [Cl(g)]}{[CFCl_3(g)]}=\dfrac{k_1}{k_{-1}}](/tpl/images/0586/7481/99f0e.png)


