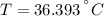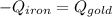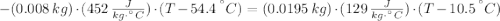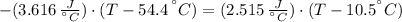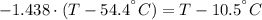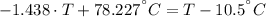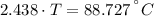Chemistry, 08.04.2020 02:07 tleppek6245
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weighing 19.5 g and at a temperature of 54.4°C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A sheet of gold weighing 8.8 g and at a temperature of 10.5°C is placed flat on a sheet of iron weig...
Questions


Social Studies, 26.06.2019 21:30

History, 26.06.2019 21:30

Mathematics, 26.06.2019 21:30

English, 26.06.2019 21:30


Mathematics, 26.06.2019 21:30



History, 26.06.2019 21:30


Mathematics, 26.06.2019 21:30


Mathematics, 26.06.2019 21:30


Mathematics, 26.06.2019 21:30


Mathematics, 26.06.2019 21:30

Mathematics, 26.06.2019 21:30

History, 26.06.2019 21:30