
Chemistry, 08.04.2020 03:32 LordBooming
For which of the following reactions is S° > 0. Choose all that apply. NH4I(s) NH3(g) + HI(g) CH4(g) + H2O(g) CO(g) + 3H2(g) 2NH3(g) + 3N2O(g) 4N2(g) + 3H2O(g) 2NH3(g) + 2O2(g) N2O(g) + 3H2O(l) CO(g) + Cl2(g) COCl2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3



Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
For which of the following reactions is S° > 0. Choose all that apply. NH4I(s) NH3(g) + HI(g) CH4...
Questions

Mathematics, 20.09.2019 01:20



Mathematics, 20.09.2019 01:20













History, 20.09.2019 01:20



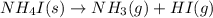
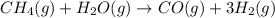
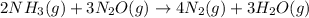
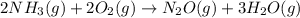
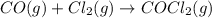
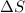 > 0.
> 0.

