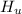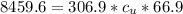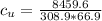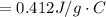Chemistry, 11.04.2020 04:47 nihadsalim10
179.1 g of water is in a Styrofoam calorimeter of negligible heat capacity. The initial T of the water is 16.1oC. After 306.9 g of an unknown compound at 94.3oC is added, the equilibrium T is 27.4oC.
What is the specific heat of the unknown compound in J/(goC)?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
179.1 g of water is in a Styrofoam calorimeter of negligible heat capacity. The initial T of the wat...
Questions

Mathematics, 23.08.2019 05:10

Mathematics, 23.08.2019 05:10

English, 23.08.2019 05:10

Mathematics, 23.08.2019 05:10


Mathematics, 23.08.2019 05:10




Mathematics, 23.08.2019 05:10


Computers and Technology, 23.08.2019 05:10



Mathematics, 23.08.2019 05:10





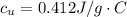
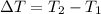
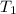 and 27.4°C for
and 27.4°C for 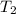
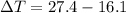
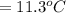
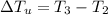
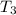
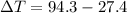
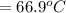
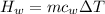
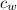 (specific heat of water)
(specific heat of water)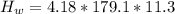
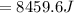
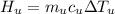
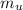 , 8459.6J for
, 8459.6J for