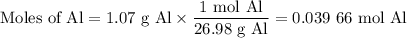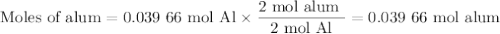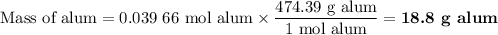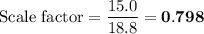Chemistry, 14.04.2020 18:53 sierravick123owr441
Scaled Synthesis of Alum. Show your calculations for:a. the experimental scaling factor giving rise to a 15.0 g theoretical yield;b. the corrected volumes of KOH and H2SO4; andc. the theoretical yield of alum based on the actual amount of Al used. Make sure you carefully show each step for these calculations.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Scaled Synthesis of Alum. Show your calculations for:a. the experimental scaling factor giving rise...
Questions

Mathematics, 07.09.2019 15:10

Mathematics, 07.09.2019 15:10

Physics, 07.09.2019 15:10

Mathematics, 07.09.2019 15:10









Biology, 07.09.2019 16:10


Biology, 07.09.2019 16:10

Biology, 07.09.2019 16:10

Biology, 07.09.2019 16:10


Biology, 07.09.2019 16:10