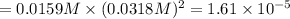PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)

Chemistry, 15.04.2020 00:52 genyjoannerubiera
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Suppose you add 0.2307 g of PbCl2 (s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of Pb2+ (aq) is 0.0159 M and the concentration of Cl− (aq) is 0.0318 M.
What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Questions


Mathematics, 14.02.2022 05:40



Mathematics, 14.02.2022 05:40


Mathematics, 14.02.2022 05:40

Biology, 14.02.2022 05:40






Biology, 14.02.2022 05:40

Mathematics, 14.02.2022 05:40



English, 14.02.2022 05:40

Mathematics, 14.02.2022 05:40

Mathematics, 14.02.2022 05:40

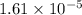 .
.![[Pb^{2+}]=0.0159 M](/tpl/images/0600/3825/89ec5.png)
![[Cl^-]=0.0318 M](/tpl/images/0600/3825/75929.png)
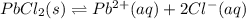
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0600/3825/7fd11.png)