Chemistry, 15.04.2020 15:52 crystalclear99
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000 M NaoH solution. Calculate the volume of NaoH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.vo...
Questions





Mathematics, 21.09.2021 23:30




Mathematics, 21.09.2021 23:30



Geography, 21.09.2021 23:30

Mathematics, 21.09.2021 23:30


Mathematics, 21.09.2021 23:30

Biology, 21.09.2021 23:30

Advanced Placement (AP), 21.09.2021 23:30

Mathematics, 21.09.2021 23:30

Mathematics, 21.09.2021 23:30

Business, 21.09.2021 23:30

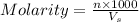
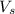 = volume of solution in ml = 250 ml
= volume of solution in ml = 250 ml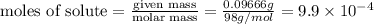
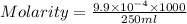
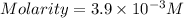
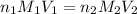
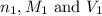 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 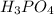
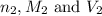 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.