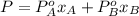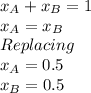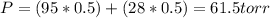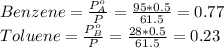Chemistry, 15.04.2020 20:08 lilakatedancer
Benzene and toluene form ideal solutions. Consider a solution of benzene and toluene prepared at 25 ^ { \circ } \mathrm { C }25∘C. Assuming that the mole fractions of benzene and toluene in the vapor phase are equal, calculate the composition of the solution. At 25 ^ { \circ } \mathrm { C }25∘C the vapor pressures of benzene and toluene are 95 and 28 torr, respectively.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3


Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Benzene and toluene form ideal solutions. Consider a solution of benzene and toluene prepared at 25...
Questions

Physics, 08.12.2020 03:00



Chemistry, 08.12.2020 03:00




Biology, 08.12.2020 03:00





Mathematics, 08.12.2020 03:00



Mathematics, 08.12.2020 03:00


Health, 08.12.2020 03:00