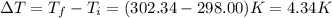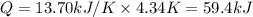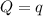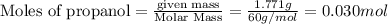Chemistry, 16.04.2020 01:46 maggie123456751
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter from 298.00 K to 302.34 K . The heat capacity of the bomb calorimeter is 13.70 kJ/K . Determine Δ H for the combustion of propanol to carbon dioxide gas and liquid water.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter...
Questions










Social Studies, 19.02.2020 22:20


Mathematics, 19.02.2020 22:20








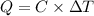
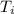 = 298.00 K
= 298.00 K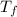 = 302.34 K
= 302.34 K