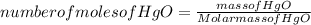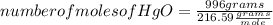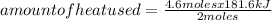Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1
You know the right answer?
A mercury mirror forms inside a test tube as a result of the thermal decomposition of mercury(ii) ox...
Questions

History, 17.01.2020 00:31

Mathematics, 17.01.2020 00:31

English, 17.01.2020 00:31

Mathematics, 17.01.2020 00:31

Social Studies, 17.01.2020 00:31

Social Studies, 17.01.2020 00:31

Advanced Placement (AP), 17.01.2020 00:31






Mathematics, 17.01.2020 00:31







Mathematics, 17.01.2020 00:31